Overview
Academic research usually unfolds in calm, controlled conditions, precise methods, long peer reviews, and cautious publication timelines.
But what happens when the world itself becomes unstable?
During pandemics, earthquakes, or wars, science must act fast. The laboratory moves to the field; the researcher becomes a first responder.
This article explores how crises reshape research, from disrupted data collection and ethical tension to rapid publishing, and offers practical strategies to maintain integrity, accuracy, and compassion when time and safety are at risk.
Methodological Challenges and the Disruption of Data Collection
Crises can paralyze traditional data collection. During COVID-19, lockdowns halted in-person trials, and researchers faced empty clinics and unreachable participants.
The challenge? To stay scientific without the usual tools.
Digital interviews
Instead of physical surveys.
Secondary data analysis
Instead of new field studies.
Online focus groups
To replace classroom or clinical observations.
The environment itself becomes unstable — affecting both reliability and reproducibility.
Ethical Challenges: Balancing Humanitarianism and Necessity
In crises, the researcher often meets participants not as subjects but as survivors.
This raises questions that standard ethical forms rarely answer:
- Can displaced or grieving individuals truly give informed consent?
- Does documenting trauma risk deepening it?
- When does “collecting data” become “exploiting suffering”?
To navigate this, modern ethics frameworks introduced tools like Rapid Ethics Review, allowing expedited but still rigorous approval in emergencies.
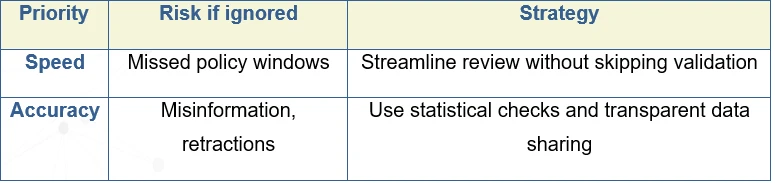
Time Pressures: The Tension Between Speed and Accuracy
Crises compress timelines. Governments and hospitals need evidence now.
During COVID-19, journal submissions increased by over 400%, flooding peer review systems and causing uneven quality control.
The tension is clear:
Strategies for Effective Research and Responsible Publishing
1. Adapting Methods Without Losing Rigor
Flexibility is survival. Use:
- Secondary data or institutional records when field access fails.
- Short cross-sectional online surveys for rapid insights.
- Free digital tools (Google Forms, KoboToolbox, Zoom) to reach participants remotely.
Constraint can fuel creativity — turning limitation into innovation.
2. Scientific Collaboration and Data Sharing
Crises prove that knowledge grows through openness.
- GISAID allowed real-time tracking of COVID-19 mutations.
- After the Turkey–Syria earthquake (2023), volunteer academics created shared databases to record damage and psychological outcomes.
For isolated researchers, virtual collaboration offers inclusion in global science without leaving home.
3. Choosing the Right Publication Path
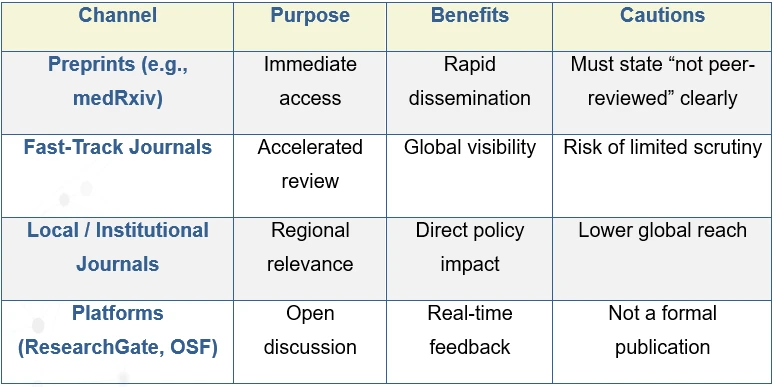
Transparency remains your armor. State limitations openly; uncertainty is more credible than overconfidence.
Crises as Open Laboratories: Lessons from the Field
1. The Pandemic: Between Knowledge Abundance and Misuse
COVID-19 created research “infodemic.” While rapid preprints accelerated discovery, they also spread unverified claims — such as the later-retracted “HIV similarity” paper.
→ Lesson: Speed without review breeds mistrust.
2. The Earthquake: Research from Under the Rubble
After the Turkey–Syria earthquake, local academics acted as first responders — collecting data on casualties, PTSD, and infrastructure damage without funding or stable labs.
→ Lesson: Local capacity and community trust are the real infrastructure of crisis research.
3. The Overarching Lessons: From Research to Awareness
Every crisis reshapes science. The researcher becomes:
- A witness as well as an analyst.
- A participant in collective survival.
- A guardian of truth amid chaos.
Common Mistakes
- Treating crisis data as normal data without documenting context.
- Publishing speed over quality or ethics.
- Ignoring local researchers in global collaborations.
- Failing to state data limitations or sample bias.
- Using preprints as “final” references.
Key Takeaways
- Crises transform research into an emergency service for truth.
- Flexibility + transparency sustain scientific integrity under pressure.
- Ethical care must never be sacrificed for speed.
- Collaboration turns isolation into impact.
- Every disaster tests — and strengthens — the culture of science.
Frequently Asked Questions (FAQs)
- How can researchers balance speed and accuracy during emergencies?
By streamlining, not skipping, scientific safeguards. Effective approaches include rapid “red-flag” reviews, basic statistical checks, transparent data sharing, and explicitly stating uncertainty and methodological constraints before publication. - Are alternative data collection methods still scientifically valid?
Yes, if used transparently and rigorously. Secondary data analysis, online surveys, and digital interviews can produce valid insights when limitations are clearly documented and methods are adapted thoughtfully rather than improvised. - How should researchers choose the right publication route during emergencies?
The choice depends on the goal: Preprints for speed and early visibility, fast-track journals for rapid peer review, local journals for regional policy impact, open platforms for discussion and feedback, transparency about limitations is essential in all cases. - Are preprints safe to use during global crises?
Preprints are valuable for rapid knowledge sharing but must be clearly labeled as not peer-reviewed. They should inform discussion and hypothesis generation, not be treated as definitive evidence or policy foundations. - Who should read this article?
Early-career researchers, clinicians, public health professionals, editors, and policymakers working in unstable or emergency settings, especially those involved in rapid evidence generation and publication.
A Word from ResRef
Global crises place science under exceptional pressure, demanding speed without surrendering rigor or ethics. This article highlights how researchers can respond responsibly by adapting methods transparently, safeguarding participant dignity, and choosing publication pathways with care. At ResRef, we believe that crisis-time research is not only about producing evidence quickly, but about preserving trust, integrity, and scientific accountability when they matter most.
References
- Horton R. (2020). Offline: COVID-19 and the dangers of fake science. The Lancet.
- Artino A.R. et al. (2014). Developing questionnaires for educational research. Medical Teacher, 36(6).
- WMA Declaration of Helsinki (2013). Ethical Principles for Medical Research Involving Human Subjects.
- Rattray J., & Jones M.C. (2007). Essential elements of questionnaire design and development. J Clin Nurs, 16(2), 234–243.
- WHO (2020). Ethics and COVID-19: Resource Allocation and Priority Setting.
A bilingual PDF (Arabic | English), containing the same information in an organized format, is available for download [here].
Authorship and Contributions
The following section acknowledges the individuals who contributed to the authorship, editing, translation, and preparation of this article, ensuring its academic integrity and clarity.

Dr. Rawan Daaboul
Author

Dr. Muhammad Ezzat Alaktaa
Editor
Consultant Orthopedic Surgeon, Director General of Damascus Hospital.

Dr. Muhammad Ezzat Alaktaa
Editor
- Phone:+963 988 525 180

Lamyaa Okko
Translator & Formatter
Occupational Therapy Student, ResRef's Website and SEO Team Member

Lamyaa Okko
Translator & Formatter
- Email:lamyaaokko751@gmail.com