Overview
How many times have you opened a survey only to abandon it halfway—too long, too
confusing, or full of vague questions?
In research, that’s not just an inconvenience; it’s a threat to data integrity and scientific
credibility.
A questionnaire is a powerful research tool—but only when designed correctly. This guide
helps you master both the art and science of questionnaire design, ensuring your data are
reliable, analysable, and truly meaningful.
From Idea to Instrument: Defining Your Research Goals
Before writing even one question, ask yourself two things:
- What exactly do I need to know?
Every item in your survey must directly connect to your research objective.
For instance, a study on job satisfaction should break down broad ideas into
measurable dimensions like salary, teamwork, and career growth. - How can I keep respondents engaged?
Keep surveys concise, relevant, and logically structured. A well-organized flow feels like a conversation, not an interrogation.
Understanding the Structure of a Questionnaire
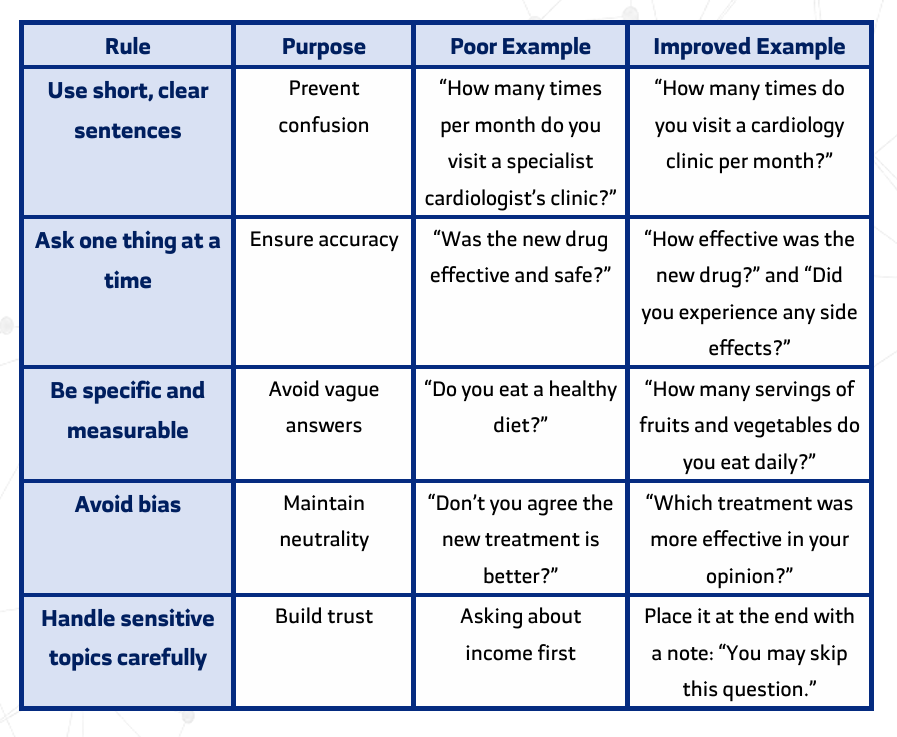
Golden Rules for Writing Questions

Question Types and Common Pitfalls
Main Question Types
- Categorical: “What is your gender?” (Male/Female)
- Likert Scale: “How satisfied are you with your job?” (1 = Very Dissatisfied → 5 = Very
Satisfied) - Semantic Differential: “Rate your hospital experience: Satisfying or Unsatisfying”
- Checklist: “Which apps do you use for medical study?” (ResRef / PubMed / Google
Scholar / Others) - Ranking: “Rank the following by usefulness: textbooks, online courses, mentorship.”
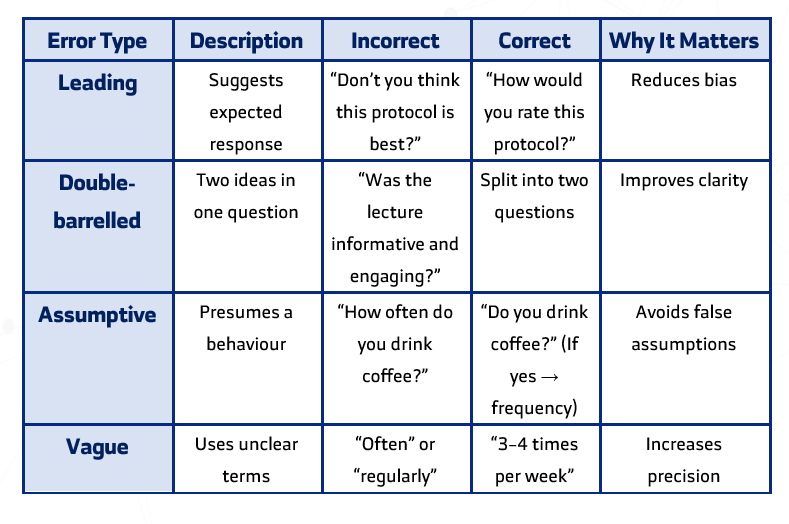
Common Wording Problems and Fixes
Ethics in Questionnaire Design
Ethical responsibility goes hand-in-hand with methodological rigor.
- Informed consent: Explain purpose, duration, and data use.
- Confidentiality: Clarify if responses are anonymous or coded.
- Non-maleficence: Avoid distressing or overly personal items.
- Ethical approval: Even online surveys often require an institutional review or exemption.
Key Takeaways
- A good questionnaire starts with clear objectives and measurable variables.
- Use simple, neutral, and focused wording.
- Always pilot test before collecting real data.
- Ethical transparency builds trust and improves response rates.
- Remember: Good data begin with good design.
Common mistakes
- Using too many open-ended questions in quantitative surveys.
- Forgetting to balance positive and negative items.
- Ignoring pilot feedback.
- Copying a tool without checking its validation source.
- Assuming participants interpret words exactly as you do.
A Word From ResRef
Well-designed questionnaires are the foundation of reliable and interpretable research findings. This guide emphasizes that clarity of objectives, thoughtful question construction, and careful testing are essential to minimizing bias and maximizing data quality. At ResRef, we encourage researchers to approach questionnaire design as a scientific process—one that requires planning, validation, and ethical responsibility to ensure meaningful and trustworthy results.
Frequently Asked Questions (FAQ)
- What is the most important step before writing questionnaire questions?
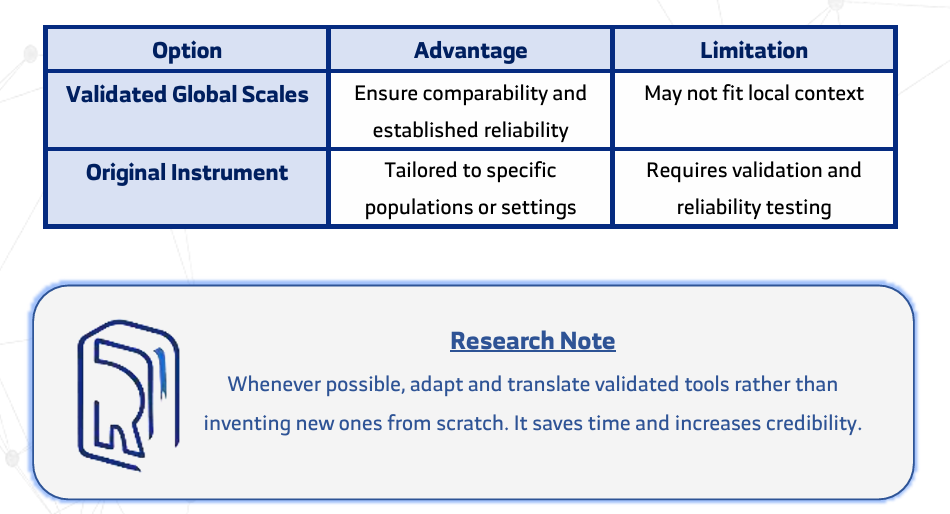
Before drafting any questions, researchers must clearly define their research objectives. Every questionnaire item should directly serve a specific, measurable research goal to ensure data relevance, clarity, and analytical value. - Should researchers use validated questionnaires or design new ones?
Whenever possible, adapting and translating validated global scales is recommended. Validated tools offer proven reliability and comparability, while newly designed instruments require additional testing for validity and reliability. - What are the most common mistakes in questionnaire question design?
Common errors include asking double-barrelled questions, using leading or biased wording, relying on vague terms, and assuming participant behaviors. These mistakes reduce accuracy and increase response bias. - Why is pilot testing essential before launching a questionnaire?
Pilot testing helps identify unclear questions, technical issues, and timing problems before data collection begins. It improves response quality, reduces missing data, and protects the overall validity of the study.
References
- Dillman, D.A., Smyth, J.D., & Christian, L.M. (2014). Internet, Phone, Mail, and Mixed
Mode Surveys: The Tailored Design Method (4th ed.). Wiley. - Artino, A.R. et al. (2014). Developing questionnaires for educational research. Medical Teacher, 36(6), 463–474.
- Rattray, J., & Jones, M.C. (2007). Essential elements of questionnaire design and
development. Journal of Clinical Nursing, 16(2), 234–243. - Brace, I. (2018). Questionnaire Design (3rd ed.). Kogan Page.
- Tourangeau, R. et al. (2000). The Psychology of Survey Response. Cambridge
University Press.
Authorship and Contributions
The following section acknowledges the individuals who contributed to the authorship, editing, translation, and preparation of this article, ensuring its academic integrity and clarity.

Dr. Rawan Daaboul
Author
M.D. and Scientific Researcher leading 28 projects; Human Resources Director at ResRef.

Dr. Rawan Daaboul
Author

Dr. M. Luay Alkotob
Editor
Interventional Cardiologist and Associate Clinical Professor at Michigan State University.

Dr. M. Luay Alkotob
Editor

Dr. Taha Al Khayrat
Translator & Formatter
A fifth-year medical student contributes to the Educational and Web departments at ResRef.

Dr. Taha Al Khayrat
Translator & Formatter