Welcome!
Understanding and writing a Case Series in medical research is more than simply collecting patient data — it’s about recognizing clinical patterns, identifying emerging diseases, and sparking the ideas that lead to future research. This guide to Case Series and Beyond will help you understand what case series are, how they are designed, and why they remain an essential foundation in medical discovery.
Moreover, by exploring this guide, you’ll learn how to structure and report a case series effectively, interpret its strengths and limitations, and apply it ethically and transparently in your own research.
Overview
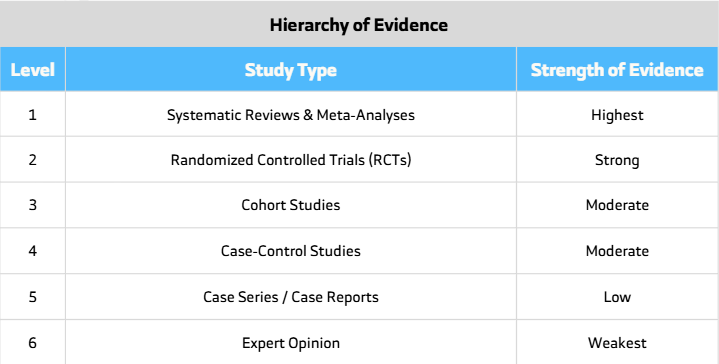
A case series is one of the simplest yet most valuable types of medical research. It describes a group of patients who share a common disease, exposure, or treatment. While it may hold a lower position in the hierarchy of evidence, its insights can be significant.
Understanding Case Series
A case series is a descriptive study that reports the clinical details of several patients with a similar condition. It lacks a control group, which means it cannot prove cause and effect.
🔹 Think of a case series as a detailed police report about a set of similar crimes. It records what happened, who was involved, and the outcomes—but it doesn’t explain why those crimes happened.
💡 Practical Tip:
Start with case series when exploring new or rare diseases. They are an essential first step before moving to higher-level studies.
Study Design and Methodology
Selection of Cases
Researchers select patients based on the presence of a particular disease or exposure. There is no control group, which makes it purely descriptive.
Data Collection
- Retrospective: Using past records or databases to identify and describe cases.
- Prospective: Identifying cases ahead of time and following them into the future.
Key Variables in a Case Series
- Demographics: Age, gender, ethnicity.
- Clinical Features: Symptoms, severity, and course of disease.
- Diagnostics: Laboratory or imaging results.
- Treatment: Procedures or medications used.
- Outcomes: Recovery, complications, or mortality.
📘 Research Note:
Always report data consistently using structured tables or timelines. This increases the credibility and reproducibility of your study.
Strengths and Limitations
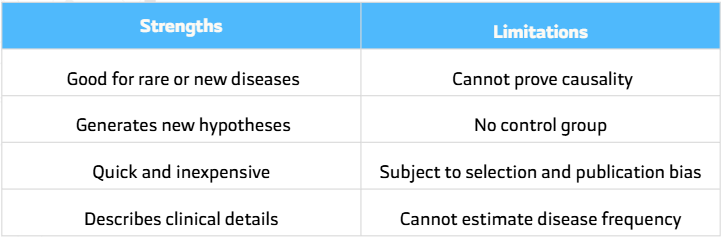
Strengths
- Useful for Rare Conditions: Often the only possible design for new or unusual diseases.
- Hypothesis-Generating: Helps identify early patterns or associations.
- Low Cost & Feasible: Especially when using existing data.
- Clinically Rich: Provides detailed, real-life patient data.
- Technique Evaluation: Demonstrates initial success of new surgical or medical methods.
Limitations
- No Control Group: Cannot confirm whether observed outcomes are truly related to the exposure.
- Bias Risks:
- Selection Bias – Patients may come from a single specialized hospital.
- Publication Bias – Only interesting or positive cases are reported.
- No Causality: Cannot prove cause-and-effect.
- No Incidence or Prevalence Data: Since it lacks a comparison population.
💡 Practical Tip:
Use a case series to inspire future analytical studies such as cohort or case-control designs.
When Case Series Shine
1. Understanding Disease Progression
In rare diseases, following several patients over time helps define the natural history of illness.
Example: Mapping progression of Duchenne muscular dystrophy from diagnosis to late complications.
2. Detecting New Risks
The link between thalidomide and birth defects, or between DES exposure and vaginal cancer, first appeared in case series.
3. Introducing Innovations
The earliest reports of heart transplants and robotic surgeries were published as case series, proving feasibility before large-scale trials.
📘 Research Note:
Case series are the starting point of discovery—they raise questions that drive science forward.
Reporting Case Series (CARE Guidelines)
Follow the CARE Guidelines to ensure clarity and transparency:
1. Abstract: Concise summary of the study.
2. Introduction: Why this case or series is unique.
3. Patient Information: Demographic and clinical details.
4. Timeline: Key events from presentation to outcome.
5. Diagnostic Assessment: Tests used to confirm diagnosis.
6. Therapeutic Intervention: Treatments provided.
7. Follow-Up and Outcomes: What happened to the patients.
8. Discussion: Literature review and interpretation.
💡 Practical Tip:
Use patient timelines or flowcharts to make your report visually engaging and easier to follow.
Common Mistakes
- Confusing a case series with a cohort study.
- Failing to include complete patient data.
- Overstating conclusions or implying causality.
- Ignoring ethical approval or patient consent.
- Not following CARE reporting standards.
Key Takeaways
- Case series are descriptive, not analytical.
- They play a crucial role in early discovery and innovation.
- Always report transparently and avoid overstating results.
- Use them as a foundation for more rigorous research designs.
Frequently Asked Questions (FAQ)
1. What is a case series in medical research?
A case series is a descriptive study that presents the clinical details of multiple patients who share a similar condition, exposure, or treatment. Unlike analytical studies, it lacks a control group and therefore cannot determine cause-and-effect relationships. However, it provides valuable insights that often lead to further hypotheses and more advanced research designs.
2. Why are case series important in clinical research?
Case series play a foundational role in medical discovery. They are often the first step in recognizing new diseases, identifying unexpected drug effects, or evaluating innovative treatment techniques. In particular, when dealing with rare or emerging conditions, case series may represent the only feasible study design available, offering essential early evidence that can guide future research.
3. What are the strengths and limitations of a case series?
Case series are practical, low-cost, and rich in clinical detail, making them ideal for exploring rare conditions or new interventions. However, the absence of a control group limits their ability to prove causation or estimate disease frequency. They are also prone to selection and publication bias, so results should be interpreted cautiously and used mainly to generate hypotheses rather than confirm them.
4. How can researchers ensure high-quality and ethical case series reporting?
Researchers should follow the CARE Guidelines, which emphasize transparency and structure in clinical case reporting. This includes providing complete patient information, obtaining informed consent, presenting a clear timeline, and discussing outcomes objectively. Ethical reporting not only protects patient privacy but also enhances the scientific credibility and reproducibility of the study.
A Word From ResRef
Case series may appear simple, yet they hold immense value in shaping the progress of medical science. Many of the world’s greatest discoveries—from identifying new syndromes to recognizing unexpected drug reactions—began as small, well-documented case series. By carefully analyzing and presenting these early observations, researchers transform bedside experiences into the foundation for larger studies and clinical breakthroughs.
In our next article, we will guide you through the process of Publishing Case Reports, highlighting how to choose the right journal, meet ethical requirements, and present your findings effectively to a global audience.
A bilingual PDF (Arabic | English), containing the same information in an organized format, is available for download [here].
Authorship and Contributions
The following section acknowledges the individuals who contributed to the authorship, editing, translation, and preparation of this article, ensuring its academic integrity and clarity.

Dr. Yasser MHD Kheir Alghabra
Author
- Phone:+963947046211
- Email:yassergh589@gmail.com

Dr. Abdulmajeed MHD Yousfan
Editor
- Phone:+963955218264
- Email:dr.yosfan@gmail.com

Dr. Mohammad Baraa Ahmad Abu Bakr
Translator & Formatter
- Phone:+963968932744
- Email:baraaab16@gmail.com
















2 thoughts on “Case Series and Beyond”
This made me feel included, not overwhelmed
Thanks for sharing. I read many of your blog posts, cool, your blog is amazing!!