Welcome to the next step of your research journey!
In this guide, we’ll explore the heart of every scientific investigation — the research process itself. You’ll learn how researchers turn simple questions into powerful studies, how they collect and analyze data, and how these results eventually improve patient care.
By the end, you’ll see that research isn’t just about experiments — it’s about asking the right questions, thinking critically, and using evidence to make better medical decisions.
From Idea to Reality: How to Build Research That Leaves an Impact
So far, we’ve learned the fundamentals of research. But how does a simple question turn into a published study that can change medical practice? The research process is a journey with several distinct stages. It requires careful planning, hard work, and attention to detail. Let’s walk through the entire path, from that first spark of an idea to the final, published discovery.
The 5 stages of Research Journey
- Finding Your Idea and Doing Your Homework
- Making a Plan (The Research Protocol)
- Doing the Study (Data Collection)
- Analysing and Interpreting the Results
- Writing and Publishing Your Findings.
Stage one: Finding Your Idea and Doing Your Homework
Every great research project starts with an idea. But where do these ideas come from?
They often emerge from a blend of observation, critical thinking, and engagement with the scientific community.
Your Daily Work (Clinical Practice):
As a healthcare professional, you are on the front lines. Pay attention to:
Practical Tip: Keep a small notebook or a digital note-taking app handy to jot down these observations as they occur. Even a fleeting thought can become a research question.
Reading (Existing Literature):
The scientific literature is a goldmine of ideas. Don’t just read for facts; read critically:

Talking with Others (Collaboration):
Research is rarely a solo endeavor. Engage with:
New Technology and Methodologies:
Advances in technology often open new avenues for research:
Do Your Homework: The Literature Review
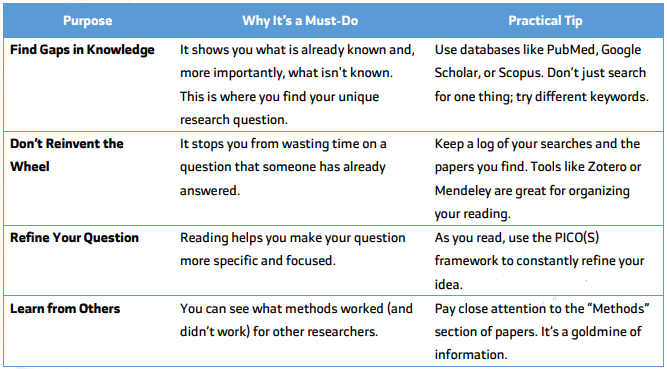
Once you have an idea, you must do a literature review. This means systematically searching for and reading everything already published on your topic. This is a critical step!
Why is a Literature Review So Important?
Stage Two: Making a Plan (The Research Protocol)
With a sharp question and a good understanding of the existing literature, it’s time to make a detailed plan. This is called a research protocol. It’s the recipe for your entire study, and it includes everything:
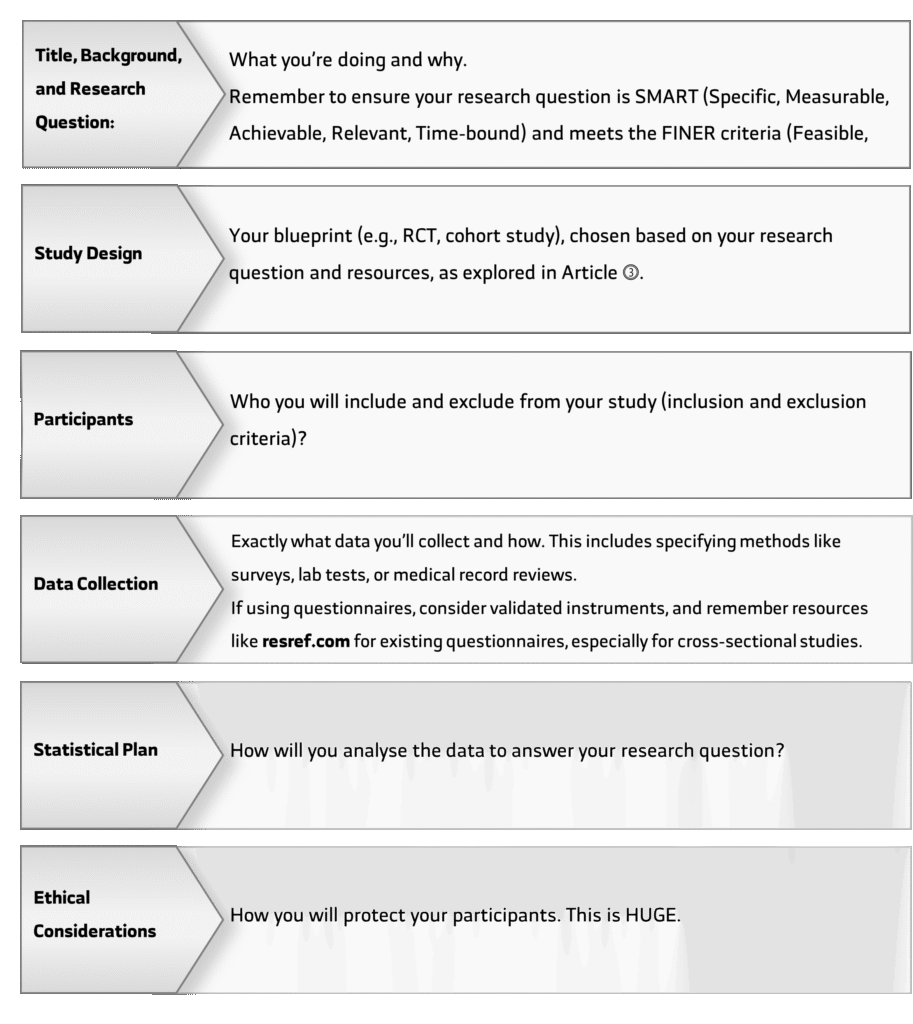
Getting the Green Light: The Ethics Committee (IRB):
Before you can start any research with humans, you must get approval from an Institutional Review Board (IRB) or Ethics Committee. Their job is to protect the rights and welfare of research participants. They will carefully review your protocol to make sure your study is ethical and safe. IRB approval is non-negotiable.
Stage Three: Doing the Study (Data Collection)
This is where your plan becomes reality. It’s often the longest phase of the project and involves:
- Recruiting and enrolling participants
- Getting informed consent (making sure participants fully understand the study before they agree to join)
- Collecting your data (doing interviews, running tests, etc.)
- Managing your data carefully — good data management is essential. Garbage in, garbage out!
Practical Tip: Create a simple data collection form and test it out before you start. This helps you catch any confusing questions or missing fields early on. Use a secure system like Redcap if it’s available to you.
Stage Four: Analysing and Interpreting the Result
Once you have your data, you’ll use the statistical plan from your protocol to analyse it. But the numbers are only half the story. Interpretation is about understanding what the numbers mean.
- Look for patterns and trends.
- See if your hypothesis was supported.
- Consider both the statistical significance (p-value) and the clinical significance (does it matter in the real world?), as discussed in Article ❷ (here).
Remember: Correlation is NOT Causation!
This is one of the most important rules in research. Just because two things are related doesn’t mean one causes the other.
Classic Example: Ice cream sales are correlated with drowning deaths. Does ice cream cause drowning? No!
The confounding variable is hot weather, which causes people to both eat more ice cream and swim more.
Stage Five: Writing and Publishing Your Findings
If research isn’t published, it’s like it never happened. Sharing your results is the final, crucial step. Most scientific papers follow a structure called IMRAD:
- Introduction: Why did you do the study?
- Methods: How did you do it?
- Results: What did you find? (Just the facts!)
- Discussion: So what? (What do your findings mean?)
After you write your paper, you submit it to a journal, where it will go through peer review. If it’s accepted, it will be published for the world to see, contributing to the ever-growing body of scientific knowledge.
Conclusion
The research process is a long but rewarding journey. It transforms a simple moment of curiosity into a piece of evidence that can improve the lives of patients. Now that you understand the whole process, you’re ready for the final, and perhaps most important, skill for any healthcare professional: how to critically read and evaluate the research of others.
Frequently Asked Questions (FAQ)
How does the scientific method guide medical research?
The scientific method provides a structured approach to research, ensuring that questions are investigated systematically. It helps researchers formulate hypotheses, design experiments, collect reliable data, and draw valid conclusions that can improve patient care.What steps are involved in designing a medical study?
Designing a medical study starts with identifying a clear research question, conducting a thorough literature review, and creating a research protocol. Ethical approval, participant recruitment, and careful planning of data collection methods are also essential to ensure trustworthy results.Why is critical thinking important in research?
Critical thinking allows researchers to analyze evidence objectively, question assumptions, and identify potential biases. This ensures that conclusions are based on facts rather than opinions, making research findings more credible and clinically useful.How do researchers ensure their results are reliable?
Reliability comes from proper study design, accurate data collection, and robust statistical analysis. Repeating experiments, using control groups, and peer review further ensure that findings are reproducible and valid for broader clinical application.
A Word From ResRef
You’ve just completed the journey from a simple idea to published research. Understanding how to build research that leaves an impact is just the beginning.
In our next article, “The Medical Detective’s Toolkit: How to Read a Research Paper and Spot the Truth,” we’ll focus on the essential skill of critical appraisal. With thousands of studies published daily, you’ll learn how to systematically evaluate research, separate high-quality evidence from flawed studies, and become a true medical detective.
A bilingual PDF (Arabic | English), containing the same information in an organized format, is available for download [here].




















2 thoughts on “From Idea to Reality: How to Build Research That Leaves an Impact”
thank you you helped me a lot !
This website bridges the gap between medicine and clarity