Welcome!
Writing a Medical Case Report is more than documenting a patient’s condition — it’s a way to share unique clinical insights, highlight rare occurrences, and contribute to the broader medical knowledge. Therefore, this guide to writing a medical case report will walk you through the essential steps to prepare a clear, structured, and ethically sound report.
Moreover, by following this guide to writing a medical case report, you’ll learn how to present a patient’s case effectively, emphasize its clinical significance, and ensure your report meets the standards of peer-reviewed medical journals.
What is a Clinical Case Report?
- It is a detailed report of a patient’s symptoms, signs, diagnosis, treatment, and follow-up.
- Moreover, case reports typically describe an unusual or novel disease, symptom, clinical sign, or association between two conditions.
- In some cases, reports may include an extensive review of the relevant medical literature.
- Therefore, case reports are considered the primary entry point into research and scientific publication for physicians who may lack the time or resources to conduct large-scale studies.
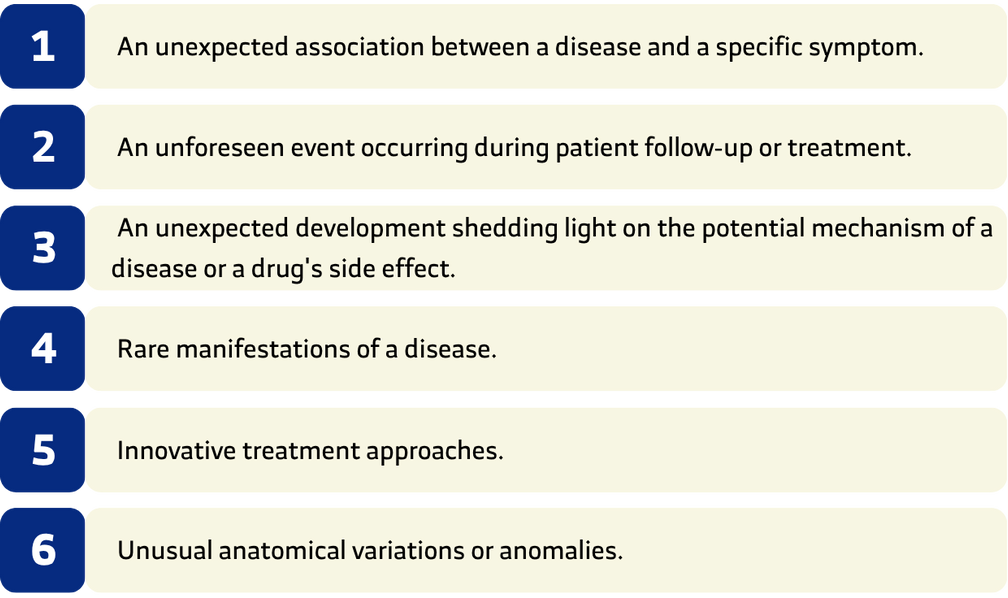
Reasons for Publishing a Medical Case Report
The most common reasons for publishing case reports are:
General Structure of a Case Report
First, it is essential to understand the general sections of a case report. However, keep in mind that each journal has its own specific guidelines for case report submissions. Nevertheless, this guide outlines the common structure followed by the vast majority of peer-reviewed medical journals.
A case report consists of the following main sections:
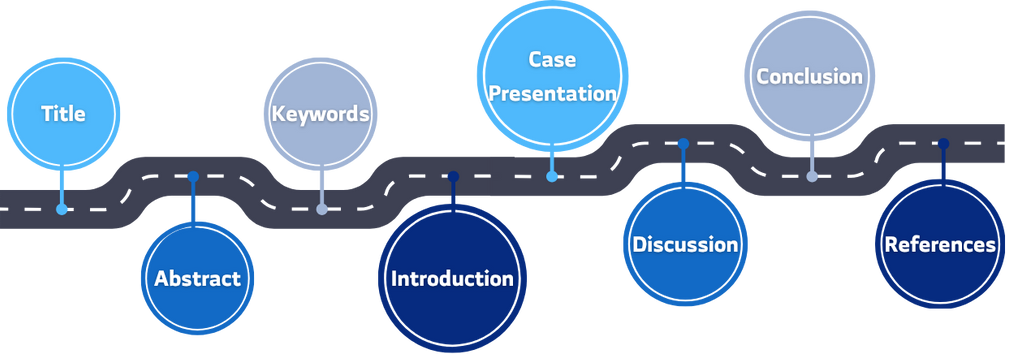
Additionally, a case report includes (listed here for completeness; details follow later):
- Author names listed in order, with affiliations.
- Email address of the corresponding author.
- Informed Consent.
- Ethical Approval.
- Acknowledgements section.
- Funding source.
- Conflict of interest statement..Data availability statement.
Title
- The title should be clear, descriptive, accurately reflecting the case content, and focused on the scientific essence.
- Additionally, avoid using abbreviations within the title.
- Conclude the title with a clarifying phrase such as: `(……..: A Case Report)` for a single patient, `(……..: A Report of Two Cases)` for two patients, or `(……..: A Case Series)` for three or more cases.
- Similarly, avoid wordplay, ambiguous phrases, or humor in the title.
- Also, avoid clichés or unscientific expressions that may diminish the research seriousness.
- Remember, the title may be the only part of your article a reader sees; it must be precise and academically appealing without ambiguity.
Practical Example: Title of a published case report by authors (ENT physicians) from Al-Mouwasat University Hospital, Damascus, concerning a young woman who developed a tragic complication.

Note: the title above contains information that allows the reader to understand the article’s general content upon first reading. Therefore, titles should avoid abbreviations. For instance, do not write “ETI” to denote Endotracheal Intubation; instead, always use the full medical term without abbreviations.
For further clarification and to illustrate common mistakes, here are examples of poor titles for the same case report:
1. Title contains abbreviations (ETI for Endotracheal Intubation):

2. Title too short:

3. Title too long:

Abstract
- The abstract is a crucial part of the report, often being the only section readers review.
- Consequently, it facilitates retrieval from electronic databases via keywords.
- It helps researchers assess their interest in reading the full report.
- Summarize the objective: Why is this case important? Then present the case, including patient information, diagnosis, and/or intervention. Finally, state the conclusion by mentioning the lessons learned or the main message of your article.
- As always, use clear scientific language and avoid uncommon abbreviations.
Characteristics of a Good Abstract:
- It should be a concise and condensed version of the full report, including the same main sections (Introduction, Case, Discussion, Conclusion).
- Write it in a brief and precise style, focusing on essential points without unnecessary details.
Practical Tip:
- Write the abstract last, after completing the full report. Ideas will flow more smoothly after sufficient thought and writing.
Example:

Details (Follow the abstract box above with explanation below):
- Why is this case important? `
We present a unique case of complete tongue necrosis caused by a compression of an endotracheal tube (ETT). As a result, it highlights critical considerations for ICU patient management.`
- Present the case: `A 39-year-old female underwent endotracheal intubation secondary to respiratory failure following sudden altered mental status. Tongue swallowing developed and worsened with obvious pallor on examination. Extensive ischemic changes with tongue necrosis developed dramatically due to the compression during her prolonged intubation. `
- State the lessons learned/main message: This case of tongue necrosis highlights the importance of proper ETT sizing and positioning during prolonged intubation in ICU patients.
- Importantly, do not include citations (references) within the abstract, as it is a summary of your article, not information quoted from another source.
- Additionally, proofread the abstract meticulously for spelling, grammar, awkward phrasing, repetition, or ambiguity.
Keywords
Keywords are essential for ensuring your article reaches the target audience through academic databases and search engines. Therefore, choose precise terms reflecting the main themes of the case, adhering to the following standards:
- Adhere to word count: The ideal number is typically between 3 to 6 keywords, depending on the publishing journal’s requirements.
- Focus on the scientific essence: State the primary diagnosis (e.g., `Lyme disease`, `Cushing’s syndrome`).
- Add distinguishing features of the case (e.g., `rare neurological complications`, `delayed diagnosis`).
- Add the distinctive therapeutic intervention if applicable (e.g., `immunotherapy`, `laparoscopic surgical resection`).
- Use standardized terminology: Employ terms recognized in medical literature, such as those from the Medical Subject Headings (MeSH) list or common terms in reputable journals.
- Balance generality and specificity: Combine broad terms (e.g., `Autoimmune Diseases`) with specific ones (e.g., `BRCA1 gene mutation`).
Introduction
- Provide a brief overview of the disease concisely, citing relevant literature when necessary.
- Furthermore, explain what makes this case unique or instructive.
- In addition, describe the knowledge gap it fills. Finally, state the objective of publishing this report (e.g., raising awareness of a rare disease).
- The introduction usually concludes with a single sentence describing the patient and the underlying condition they presented with.
- Proofread the introduction meticulously for spelling, grammar, awkward phrasing, repetition, or ambiguity.
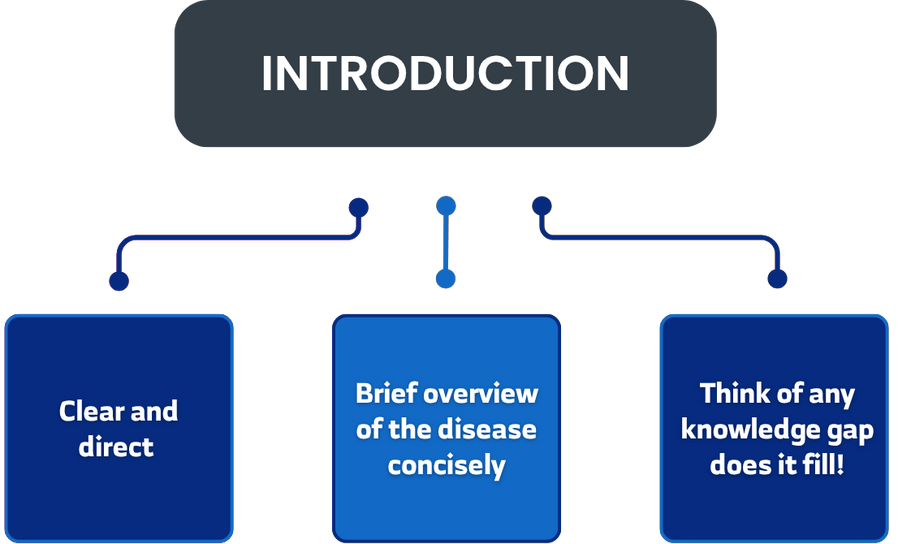
Case Presentation (The Case Report)
- Provide a detailed, organized description of the case.
- Begin by stating the patient’s demographic information: Age, gender, medical history (while maintaining patient confidentiality: Do not state the patient’s name or address – detailed later).
- Next, describe the Chief Complaint: The symptom that brought the patient to the clinic or emergency department.
- Then, outline the History of Present Illness: Detail the chief complaint and any associated symptoms if present.
- Clinical Examinatio: Report findings from the physical exam and vital signs.
- Subsequently, present Diagnostic Investigations: Results of laboratory tests, imaging (e.g., echo, CT scan, MRI, scintigraphy), biopsies, or other diagnostic procedures.
- Afterwards, discuss the Differential Diagnosis: Diseases that were ruled out.
- Then, describe the Treatment: Medications, surgery, and/or any other interventions.
- Finally, report Follow-up and Outcome: The patient’s clinical progression (improvement or deterioration) and/or complications.
- Moreover, arrange events chronologically for clarity.
- Additionally, the writer must include all relevant details while excluding unnecessary ones.
- Furthermore, proofread the case presentation meticulously for spelling, grammar, awkward phrasing, repetition, or ambiguity.
Discussion
- The discussion is the most critical part of the report, convincing the journal of the case’s importance for publication.
- First, begin by expanding on what was mentioned in the introduction, focusing on the case’s uniqueness and its connection to a scientific or clinical problem.
- Then, compare the findings with previously published literature in international peer-reviewed journals (cite previous studies).
- Subsequently follow this with a review of the existing published medical literature on the topic (if the journal requires a separate literature review section, it is added before the Discussion).
- Link the case to current literature, clarifying whether the results support or contradict the current understanding of the problem.
- In particular, discuss the case’s distinguishing aspects, for example:
- Previously unrecorded age of onset for a disease manifestation.
- Unusual findings in a specific disease.
- Difficult diagnosis.
- Unexpected response to treatment.
- Rapid, unexpected, or unusual progression of a specific condition.
- Finally, explain the lessons learned to enrich and guide future clinical practice.
- Avoid repetition; focus on analysis rather than re-narrating the case.
- Moreover, proofread the discussion meticulously for spelling, grammar, awkward phrasing, repetition, or ambiguity.

Conclusion
- Case reports are concluded either with a separate conclusion or summary points, depending on the journal’s format.
- First, clearly state the main message you want to convey to the readers of your case report.
- Next, highlight the novel contribution of this case, which distinguishes it from previously published reports.
- Moreover, explain how your article might impact clinical practice or research.
- Importantly, be as realistic as possible and avoid overgeneralizing the findings.
- Finally, you may include clinical recommendations for researchers to conduct further studies, thereby improving understanding of the case, its treatment, or related drug effects.
Note:
Some journals integrate the conclusion into the final paragraph of the Discussion section.
References
- Follow a consistent citation style (e.g., Vancouver, APA, AMA) as required by the target journal.
- Include all cited sources accurately.
Additional Essential Elements
- Author Names: List author names in order, with academic degrees and affiliations/institutions.
- Corresponding Author Email: Provide the email address of the author designated for correspondence with the journal.
- Informed Consent: A fundamental ethical requirement. Written consent must be obtained from the patient (or parent/guardian for minors, or next of kin/legal guardian for incapacitated or deceased adults) before writing the report. Importantly, proof of consent is usually required upon submission. Store the original consent form securely in the patient’s medical record and keep a copy in your files. Journals require documentation of consent.
- Ethical Approval: Approval from an Institutional Review Board (IRB) or Ethics Committee may be required, especially for sensitive cases or if part of a series. Additionally, check journal and institutional requirements.
- Acknowledgements: A section to thank individuals or institutions who contributed to the work but do not meet authorship criteria (e.g., technical assistance, language editing).
- Source of Funding: Declare any funding sources that supported the work. If none, state “None declared” or similar.
- Conflict of Interest: Authors must declare any potential conflicts of interest (financial, personal, professional) that could influence the work or its interpretation. If none exist, state “The authors declare no conflict of interest.”
- Data Availability Statement: Indicate if the data supporting the case report (e.g., de-identified patient data, imaging) are available and where/how they can be accessed, or state restrictions if applicable (e.g., patient privacy).
A full case report manuscript should therefore include several additional sections to comply with journal guidelines. For instance, these may consist of Acknowledgments, a Data Availability Statement, Informed Consent, Ethical Approval, a Declaration of Conflicting Interests, and Funding.
Author Names
- List author names in English, starting from the left and top.
- Include academic degrees and affiliation/institution.
Corresponding Author Email
- Clearly indicate the corresponding author (e.g., using an asterisk * or note) and provide their email address.
Informed Consent
Patient Anonymity:
- Protecting patient identity is paramount.
- Therefore, avoid disclosing any personal details that could lead to identification, such as: names, addresses, or specific distinguishing dates.
- Additionally, pay special attention to attached images (e.g., facial images or distinctive body parts). Ensure images of affected areas do not contain identifiable marks (like tattoos or distinctive scars).
- Always consult the target journal’s specific publication guidelines to ensure all ethical and technical requirements are met before submission.
Practical Notes from Experience:
- Patients and their families often agree to publication provided complete confidentiality is guaranteed.
- Moreover, keep copies of consent forms to facilitate the process.
Advice: Ensure consent forms are regularly updated according to the latest ethical and legal standards in your country of practice.
Dos and Don'ts
Follow “Dos and Don’ts” is crucial for writing a compelling and publishable case report. Here is a list of Dos and Don’ts for a case report study, covering everything from conception to submission.


Frequently Asked Questions (FAQ)
1. What is a medical case report?
A medical case report is a detailed description of a patient’s symptoms, diagnosis, treatment, and follow-up. Specifically, it often highlights unusual cases, rare disease presentations, or innovative interventions, which together provide valuable learning opportunities for other healthcare professionals. In addition, it allows clinicians to document experiences that may otherwise be overlooked in large-scale studies.
2. Why should physicians publish case reports?
Publishing case reports allows clinicians to share novel findings, report unexpected treatment outcomes, and contribute to medical research. Moreover, it serves as an entry point into scientific publication for those who may not have the resources for large-scale studies. Furthermore, publishing encourages reflection on clinical practice and can stimulate discussion among peers.
3. What are the key components of a case report?
A high-quality case report includes a clear title, structured abstract, introduction, detailed case presentation, discussion, conclusion, references, and essential ethical documentation, such as informed consent and conflict of interest statements. Additionally, adhering to these components ensures that the report is both comprehensive and scientifically credible. Therefore, following this structure increases the likelihood of acceptance in peer-reviewed journals.
4. How do I ensure my case report is ethical and publishable?
Always obtain informed consent from the patient or their guardian and maintain confidentiality. Moreover, follow journal guidelines meticulously, and disclose any potential conflicts of interest. In this way, ethical compliance is assured, which is critical for acceptance and professional credibility. Also, consider consulting with your institution’s ethics board if you are unsure about any aspect.
5. How can a case report impact clinical practice?
Well-written case reports can alert healthcare professionals to rare conditions, novel treatment approaches, or unforeseen complications. Consequently, they support evidence-based practice by sharing lessons learned from real-world clinical experiences. In particular, highlighting practical takeaways can influence patient care and guide future research.
A Word From ResRef
Writing a Medical Case Report is an essential skill for every clinician and researcher. By carefully documenting and analyzing individual cases, you contribute valuable insights that can improve patient care and advance medical knowledge. Ultimately, mastering this process strengthens your research skills and allows you to share important clinical lessons with the global medical community. In summary, case reports bridge clinical experience with scientific evidence, benefiting both practitioners and patients.
A bilingual PDF (Arabic | English), containing the same information in an organized format, is available for download [here].
Authorship and Contributions
The following section acknowledges the individuals who contributed to the authorship, editing, translation, and preparation of this article, ensuring its academic integrity and clarity.

Dr. Yasser MHD Kheir Alghabra
Author
- Phone:+963947046211
- Email:yassergh589@gmail.com

Dr. Abdulmajeed MHD Yousfan
Editor
- Phone:+963955218264
- Email:dr.yosfan@gmail.com

Dr. Mohammad Baraa Ahmad Abu Bakr
Translator & Formatter
- Phone:+963968932744
- Email:baraaab16@gmail.com















3 thoughts on “The Practical Guide to Writing a Medical Case Report”
This platform supports better healthcare insights
Medical forms usually confuse me, but this actually helped
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you.